MT1186-A02 (Edaravone)
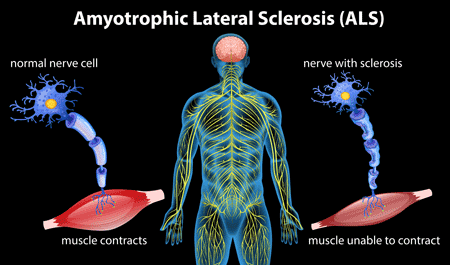
About the trial
A Phase 3b, Multicenter, Randomized, Double-Blind Study To evaluate and compare the efficacy of two dosing regimens of oral edaravone in subjects with amyotrophic lateral sclerosis (ALS) based on the change in ALS Functional Rating Scale- Revised (ALSFRS-R) score from baseline up to Week 48.
Planned enrolment is 380 participants in 95 study locations.
How DCR - CIU supports the trial
DCR - CIU is involved in multiple aspects of the trial conduct: the planning, coordination and conduct of the study visits; collection, processing, storage and shipping of biological samples, IMP dispensing and accountability, maintenance of the Investigator Site File, and data entry.
Principal Investigator
PD Dr. med. Olivier Scheidegger, Leitender Arzt, Leiter Neuromuskuläres Zentrum, Universitätsinstitut für Diagnostische und Interventionelle Neuroradiologie
Sponsor
Mitsubishi Tanabe Pharma Corporation

